What is MPS VI?
MPS VI, known as Maroteaux-Lamy disease, is one of the mucopolysaccharide storage diseases. MPS VI was first identified by Dr Maroteaux and Dr Lamy in 1963.
Mucopolysaccharides are long chains of sugar molecules used in the building of bones, cartilage, skin, tendons and many other tissues in the body. In the course of normal life there is a continuous recycling process of building new mucopolysaccharides and breaking down old ones. The breakdown and recycling process requires a series of special biochemical tools called enzymes.
People with MPS VI are missing or are low in an enzyme called N-acetylgalactosamine-4-sulfatase, which is essential in breaking down mucopolysaccharides dermatan sulphate. When dermatan sulphate is not completely broken down it remains stored in the body and the symptoms of MPS VI occur.

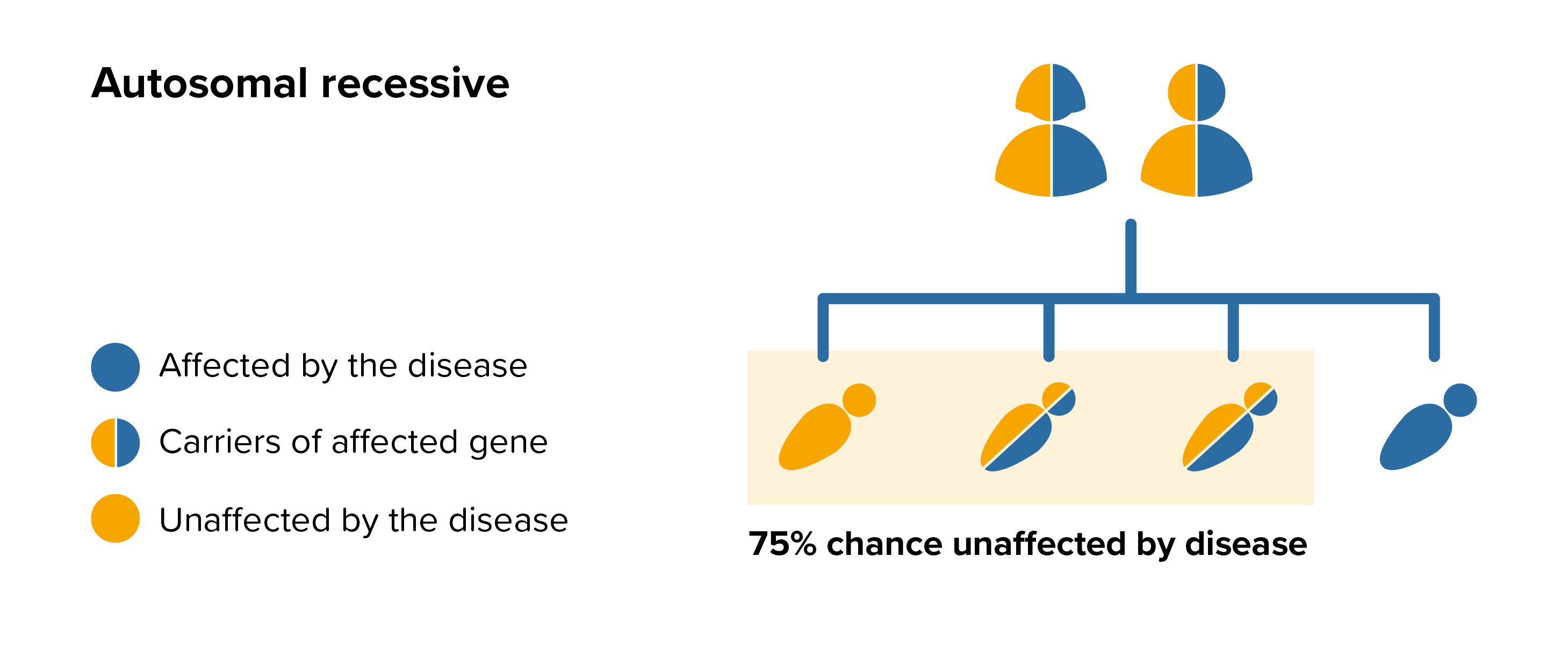 All parents of children with MPS VI can benefit from genetic counselling, the counsellor can provide advice on the risk to close relatives and to suggest whether the wider family should be informed. To find out during a pregnancy, if the baby is affected by MPS VI, screening tests can be arranged early on during a pregnancy for those families who already have a child with MPS VI. Where only one parent is a carrier, they can opt for carrier screening but it is not 100% reliable or accurate and is not possible in all cases.
All parents of children with MPS VI can benefit from genetic counselling, the counsellor can provide advice on the risk to close relatives and to suggest whether the wider family should be informed. To find out during a pregnancy, if the baby is affected by MPS VI, screening tests can be arranged early on during a pregnancy for those families who already have a child with MPS VI. Where only one parent is a carrier, they can opt for carrier screening but it is not 100% reliable or accurate and is not possible in all cases. 







