At the MPS Society we recognise that when it comes to supporting our members we can do so much more through collaboration. Each month your MPS Society Team represents you on a variety of different forums both nationally and internationally. Here’s what we’ve been up to this month.
Bob
This month Bob has travelled far and wide on MPS Society and RDRP business, all while navigating family life with a rare disease. His highlight was a trip to the USA with RDRP as these visits are crucial to promote their work and ensure that the patient voice remains central in the development and delivery of clinical trials.
Another highlight was the opportunity to meet the new Executive Director of Biomarin on their trip to London, and attending an ABPI (Association of the British Pharmaceutical Industry) meeting focusing on how to optimise engagement and involvement of rare disease patients and patient advocates. Ensuring your voices are heard is always at the top of our agenda!
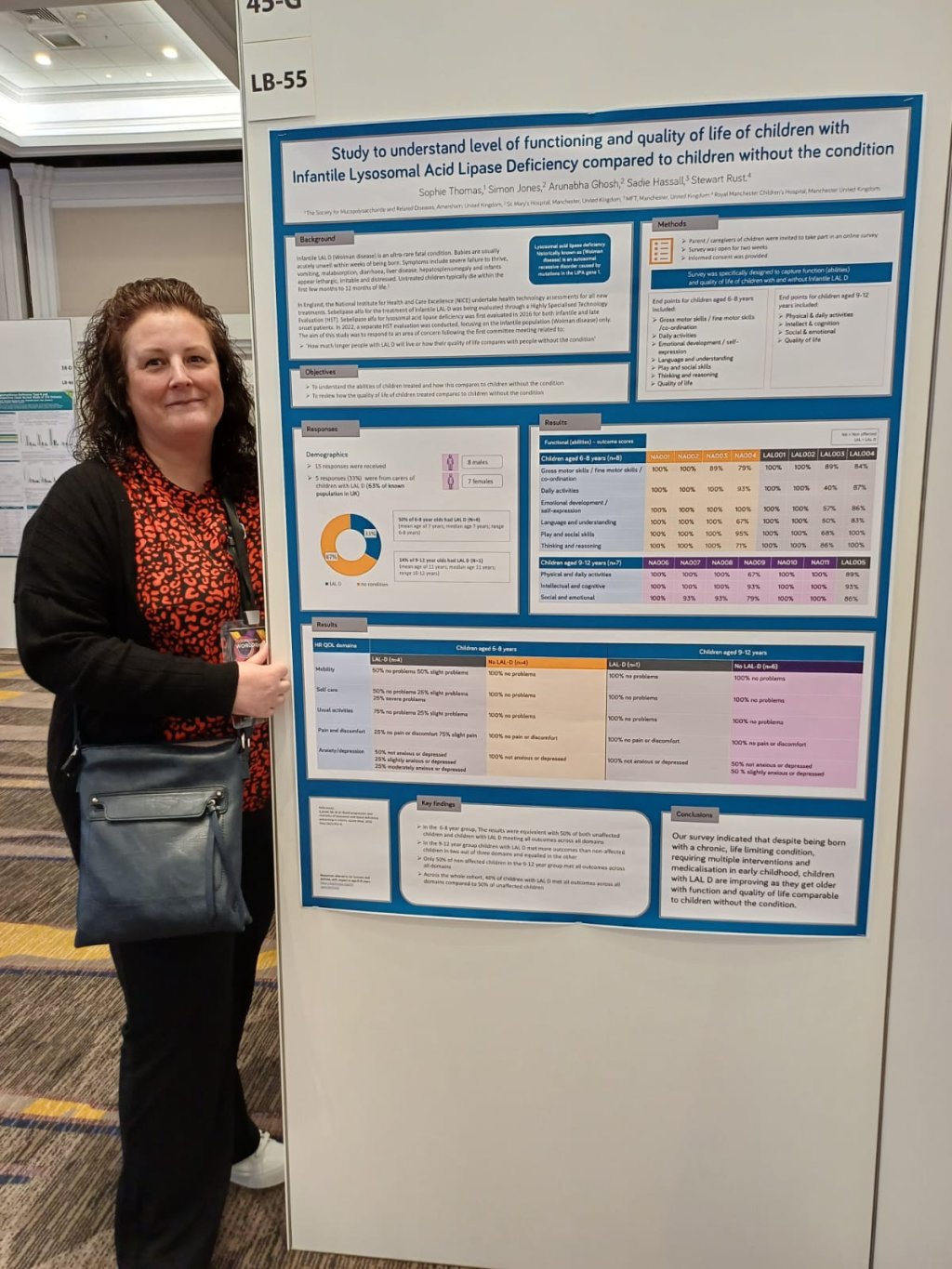
Sophie
Sophie continues to work at a high level representing your needs in forums that can and will change how the conditions we support are managed within the NHS and beyond.
Bob and Sophie met with members of the MHRA (Medicines and Healthcare products Regulatory Agency) Highly Personalised Medicines Expert Working Group to ensure their request around the need to engage with patients with lived experience when setting up working groups was heard.
Sophie has also represented the LSD Collaborative at a recent homecare supplier meeting and is engaged in our yearly meetings with the individual clinical centres.
She also continues to work across a number of areas of focus including airways, clinical guidelines, transition, cardiac services and mental health services and hopes to bring you updates on these soon.
Steve
In London, Steve attended the Specialised Healthcare Alliance (SHCA) meeting. The SHCA brings together patient organisations, charities and industry to advocate for those with rare conditions in need of specialist healthcare. The meeting reviewed progress of the Rare Disease Framework, the current challenges in clinical trials and research, commissioning and access to treatment. We also discussed the focus for 2025 which lies on seeking opportunities within the government/ NHS plan, reform access to medicines decision making, championing access to mental health services, and making the case for newborn screening. There is still lots more to do!
You can have your say on the reform of the NHS by contributing to the Change Consultation.




