Below is a patient newsletter from biotechnology company Sangamo about their Fabry gene therapy trial.

The STAAR Study is Recruiting Patients Now
Fabry disease is caused by shortage of an enzyme called alpha-galactosidase A (α-Gal A). This shortage happens when the GLA gene, which provides the body with instructions for making α-Gal A, is not working correctly.
The STAAR phase I/II clinical study has been designed to investigate the safety and tolerability of an investigational gene therapy called ST-920 to treat Fabry disease. ST-920 aims to deliver a healthy copy of the GLA gene to the liver. It is hoped that the liver should then be able to produce the α-Gal A enzyme and secrete it via the blood stream to the rest of the body.
The STAAR Study is recruiting adults aged 18 and over who have been diagnosed with Fabry disease. The first patients have received the study medication in the third quarter of 2020.
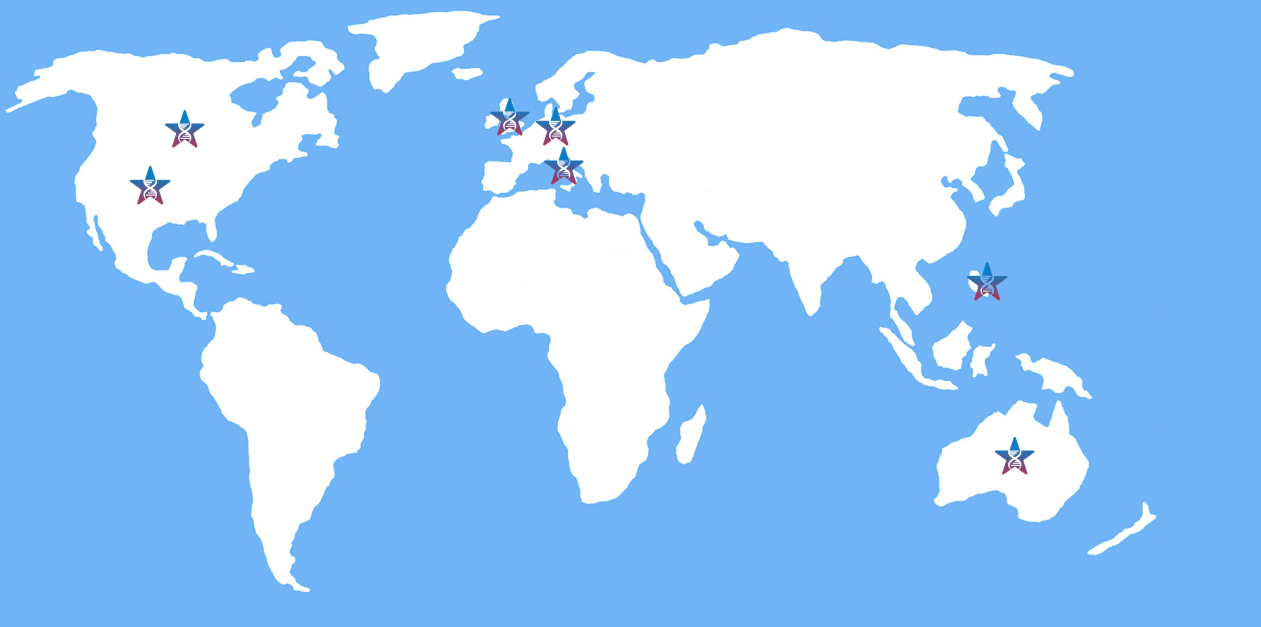
8 clinical sites are currently active in the United States and in the United Kingdom, with additional sites pending to be activated in these two countries as well as in Australia, Canada, Germany, Italy, Taiwan.
Visit the STAAR Study website where you can see if you qualify. You can also discuss this further with the study team, who are more than happy to help.
Below, you can see where STAAR clinical trials are active around the world.
More information
If you would like to get in contact with Sangamo about clinical trials, please email them here.
Click here to view our patient information sheet which highlights two of these studies and the clinical trial sites that are open in the UK.



